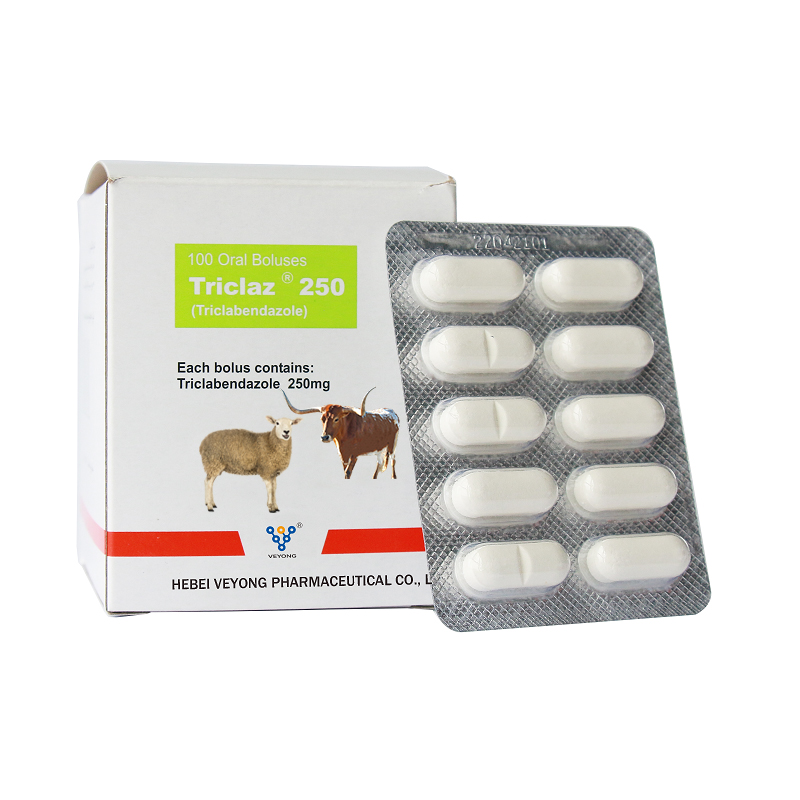
250mg Triclabendazole Bolus
AKKKỌ EGO
Promacinenacs triclobendazole nwere Benzidazole Klas nke ọgwụ ọjọọ, ọ na - eji ya eguzogide na-egbu maramara, ma nwee mmetụta doro anya na fasciola Hepational Heppica Hes. ọgwụ gluke. Mgbe ọgwụ ọgwụ a na-etinye, ọ na-egbochi ọgwụ microtubule na ọrụ nke nje ndị ọzọ, na-egbochi mwepụta nke parasiiti Hydrolytic na-eme ihe. Mmetụta nke Triclobendazole na ikpuru dịgasị na ịta ahụ, dị ka ndị okenye na ala dị ala (1 ~ 3μμ)
Ọgwụ ahụ ka dị ndụ maka awa 24, ọrụ ahụ na-esighi ike na elu ịta (10-25μG / ml) maka awa 24; Nnukwu ntinye nke 25-50μG / ml na-egbochi ya maka awa 24. Ma na-emetụta ikpuru. Na 10 μg / ml, a na-emerụ ọrụ awa 24 niile.
Ifuru Prashmashinetik
BioAViality nke Triclobendazole dị elu. Mgbe ịghasịrị nchịkwa nke 10 mg / n'arọ na ewu na atụrụ, ọnụ ọgụgụ kacha elu nke CASMA ruru 15 μg / ml na iri atọ na iri atọ na iri atọ na iri atọ na iri atọ na iri atọ na iri atọ na ya dị elu. Ọnụ ọgụgụ kachasị elu nke ọgwụ bụ ugboro 5 ruo iri abụọ nke Benzididazole ndị ọzọ anthelmidazole, na mkpochapu ọkara ndụ bụ ihe dị ka awa 22. Triclobendazole na - eme ka oxidized na atụrụ na oke to sulfone na sulfoxide derivatives, nke jikọtara ọnụ na plasma ruo ihe karịrị ụbọchị asaa. Nnukwu plasma lekwasịrị anya na njiko na plasma album na-egosi na ọ ga-ejikọ ya na oge ogologo oge nke Antifọdioli. Mgbe ụbọchị ọchịchị ọgwụ iri nke ọgwụ ọgwụ dị na atụrụ, ihe dị ka 95% nke ọgwụ a na-eme na feces, a na-emegharị ya na mmamịrị, ma na-erughị 1% na mmiri ara ehi.

Omume na iji
BenZidazole anti-faskirila. A na-ejikarị ya maka mgbochi na ọgwụgwọ nke fasciola Hepacta ọrịa na ehi na atụrụ.
Mmeghachi omume ọjọọ
Enweghị mmeghachi omume ọjọọ mgbe ejiri mmeghachi omume dị ka ojiji dị ka ojiji na usoro
Mkpachapụ anya
(1) nwere nkwarụ n'oge mmepụta mmiri ara ehi.
(2) Ọ na-egbu egbu na azụ, na akpa ọgwụ ọjọọ ekwesịghị imerụ mmiri mmiri.
(3) Ndị na-ata ahụhụ na ọgwụ kwesịrị izere ịkpọtụrụ akpụkpọ ahụ mgbe ị na-eji ha, na-eji uwe, ị na-a drinkingụ mmanya na ị smokingụ sịga.
(4) sachaa aka mgbe itinye ya n'ọrụ
Oge ndọrọ ego
Daysbọchị 56 maka ehi na atụrụ
Storaji
Chekwaa na ebe di n'okpuru 30 ℃.
Hedu Veyong Staycumcy Co., LTD, nke dị na Shijezhuan City, Hebei Provice, China, na-esote isi obodo Beijing. Ọ bụ ya buru ibu GMP na-emegwara banyere ọgwụgwọ ọgwụgwọ anụmanụ, na R & D, mmepụta na ire ahịa APA, nkwadebe maka faili na-eri nri. Dịka ụlọ ọrụ teknụzụ mpaghara, Veyong guzobere usoro R & D mepụtara ọhụrụ, ma bụrụ ndị ọkachamara na teknụzụ dị iche iche nke mba ahụ, enwere ndị ọkachamara 65. Veyog nwere ntọala abụọ na-emepụta ihe: Shinazhung Bag na ọdụ nke Shinmectin na-ekpuchi, exines, ntụ ntụ, bolucides na disinfter, ects. Veyong na-enye APIS, ihe karịrị 100 nke onwe ya na nkwadebe ya, yana ọrụ OEM & ODM.
Veyong na-etinye oke mkpa maka njikwa EHS (gburugburu ebe obibi, ahụike & nchekwa) sistemụ, ma nweta asambodo ISO14001 na Ohsas18001 asambodo. Edepụtala Veyong edepụtara na ụlọ ọrụ mmepụta ụlọ ọrụ na HEBIEI na - enwe ike ịgba mbọ hụ na ngwaahịa ngwaahịa na - aga n'ihu.

Asambodo Asambodo zuru ezu, Asambodo ISO9001, Asambodo Asambodo GMP, Atiopia Gmp Gmp, Atiopia GMP, Ahịhịa GMP, ma gafere nyocha US FDA. Veyong nwere ndị otu ọkachamara na ndebanye aha, ọrụ na ọrụ aka ọrụ, ụlọ ọrụ anyị enwetala ntụkwasị obi na nkwado ngwaahịa dị mma, ụgwọ ahịa dị elu na ọrụ ọpụpụ na nke sayensị. Veyong emeela nkwado ogologo oge na ụlọ ọrụ ọgwụ ụmụ anụmanụ ama ama na ngwaahịa ndị na-ebuga na ngwaahịa ndị a na-ebugharị na Europe, Middle Africa, wdg mba 60 na mpaghara.




.png)
.png)
.png)
.png)